1.119.843
kiadvánnyal nyújtjuk Magyarország legnagyobb antikvár könyv-kínálatát
Theoretical Aspects of Physical Organic Chemistry
The Sn2 Mechanism
| Kiadó: | Wiley-Interscience Publication-John Wiley & Sons, Inc. |
|---|---|
| Kiadás helye: | New York |
| Kiadás éve: | |
| Kötés típusa: | Fűzött keménykötés |
| Oldalszám: | 283 oldal |
| Sorozatcím: | |
| Kötetszám: | |
| Nyelv: | Angol |
| Méret: | 24 cm x 16 cm |
| ISBN: | 0-471-84041-6 |
| Megjegyzés: | Fekete-fehér ábrákkal. |
naponta értesítjük a beérkező friss
kiadványokról
naponta értesítjük a beérkező friss
kiadványokról
Fülszöveg
Of related interest
CAGE HYDROCARBONS Edited by George A. Olah
Dedicated to Paul Schleyer, whose discovery of the preparation of adamantane set the modern age of cage hydrocarbon chemistry in motion thirty years ago, this book offers a comprehensive overview of the subject—from Schleyer's early work through the most recent advances. Comprising ten chapters written by more than a dozen eminent researchers, this work comprises a representative, broad view of this dynamic and fast-growing field. 1990 (0 471-62292-3) 448 pp.
ELECTRONIC ASPECTS OF ORGANIC PHOTOCHEMISTRY
Josef Nichl and Vlasta Bonacic-Koutecky An important departure from traditional coverage of synthetic, experimental, and mechanistic aspects of the field, this book is the first work dedicated to discussion of the theoretical aspects of organic photochemistry. The focus of this work is on the analysis of the steps in terms of potential energy sources. A balanced treatment of both very simple models and advanced... Tovább
Fülszöveg
Of related interest
CAGE HYDROCARBONS Edited by George A. Olah
Dedicated to Paul Schleyer, whose discovery of the preparation of adamantane set the modern age of cage hydrocarbon chemistry in motion thirty years ago, this book offers a comprehensive overview of the subject—from Schleyer's early work through the most recent advances. Comprising ten chapters written by more than a dozen eminent researchers, this work comprises a representative, broad view of this dynamic and fast-growing field. 1990 (0 471-62292-3) 448 pp.
ELECTRONIC ASPECTS OF ORGANIC PHOTOCHEMISTRY
Josef Nichl and Vlasta Bonacic-Koutecky An important departure from traditional coverage of synthetic, experimental, and mechanistic aspects of the field, this book is the first work dedicated to discussion of the theoretical aspects of organic photochemistry. The focus of this work is on the analysis of the steps in terms of potential energy sources. A balanced treatment of both very simple models and advanced quantum chemical calculations is offered, and new insights are provided on how organic photochemical reactions occur, as well as why their products have the structures observed. 1990 (0 471-89626-8) 496 pp.
CONCEPTS AND APPLICATIONS OF MOLECULAR SIMILARITY
Edited by Marit A. Johnson and Gerald N. Naggiora This edited volume offers authoritative overviews of topics related to the definition, computation, and application of molecular similarity and emphasizes current research trends with molecular similarity as the unifying concept. The only single source available, it introduces and defines the concept of molecular similarity and explains how it can be used to explore the data containing 2-D and 3-D chemical information. The text addresses the basic problem of relating chemical structures to their associated chemical and biological properties. Final chapters illustrate the use of similarity arguments in the study of chemical reaction pathways and present theoretical approaches to the concept of molecular similarity. 1990 (0 471-62175-7) 416 pp.
WILEY-INTERSCIENCE John Wiley 6f Sons, Inc. Professional, Reference and Trade Qroup 605 Third Avenue, Flew York, N.Y. 10158-0012 riew York • Chichester • Brisbane • Toronto • Singapore
ISBN D-^7l-n4D^l-b
Q f xwu f y «aiitti >
978047184041190000
i I-
i : I
i ! ¦
Mi.!',' • : V 1 . • ¦ ^t ' •
' i ' ' i ' , ' • ¦ V. ! 'i ' ¦ 1 . i ' ^
''I 'V. ^
i' I , 1 i
V,M 1,( I ; >
Mi
, I
ll' I i, i I
if ' Ml' J : '
I'i'iV •
I'll ' ' ' i , 1.1
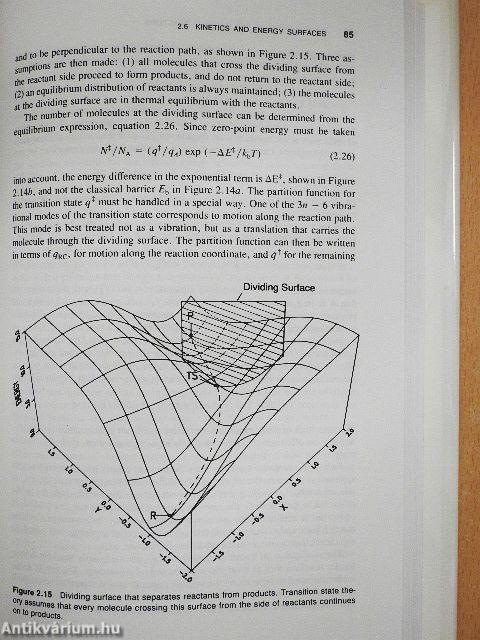
Beginning with the background concepts and historical evolution of organic chemistry, this book goes on to provide a novel model for thinking about chemical reactivity. Employing this model, the authors unify concepts in physical organic chemistry and theoretical organic chemistry. The roots of the breakdowns of these concepts are discussed along with domains of application.
The mechanism of barrier and transition state formation is discussed in a manner that facilitates predictions about barrier heights, transition state geometries and solvent effect. In addition, the authors discuss modern quantum transition state on potential energy surfaces.
As the title suggests. Theoretical Aspects of Physical Organic Chemistry: The Mechanism also presents an in-depth analysis of the S|,,2 reaction and uses It to illustrate the principles and unifying power of the model. The book shows the mechanism of barrier and TS formation for the reaction in terms of avoided crossing of VB structures. Reactivity factors are derived from simple tbermochemical quantities, such as bond strengths and group electron affinities, and are used qualitatively as predictors.
This unique book offers an excellent foundation for advanced undergraduate or introductory graduate courses in physical organic chemistry. It is also an important reference for professionals and faculty members.
About the authors
PhD m theoretical organic chemistry from the University of Wash-mgton in 1978. A Fulbright Fellow from 1974 to 1979, Professor Shaik did his postdoctoral work at Cornell University. In 1988, he received the Israel Chemical Society Award for the Outstanding Young Chemist. He is presently Professor of Theoretical Organic Chemistry at Ben Qurion University of hegev. Beer Sheva, Israel.
H. BERnnARD SCHLEGEL was awarded a PhD in theoretical organic chemistry from Queen's University, Ontario, Canada, in 1975. Since 1980 he has been Professor of Chemistry at Wayne State University, Detroit, Michigan. Professor Schlegel has been a Visiting Fellow at Princeton University {1976-77} and at Carnegie-Mellon University (1977-78). A research scientist with Merck Sharp and Dohme Research Laboratories from 1978 to 1980, Professor Schlegel has received an Alfred P. Sloan Foundation Fellowship and is a Dreyfus Foundation Teacher Scholar.
SAUL WOLFE received his PhD in organic chemistry from the University of Ottawa in 1957. University Professor and Canada Council Killam Research Fellow at Simon Fraser University, he was Chown Research Professor at Queen's University in Kingston, Ontario from 1977 to 1990. Professor Wolfe became a Fellow of the Chemical Institute of Canada in 1966, and a Fellow of the Royal Society of Canada in 1985. He received the Merck Award of the Chemical Institute of Canada in 1972, the Queen's University Prize for Excellence in Research in 1984, and the Canadian Society for Chemistry's first R. U. Lemieux Award in 1992. From 1977 to 1978 he served as Chairman of the Division of Organic Chemistry of the Chemical Institute of Canada.
f '
. ¦¦ i; i i r Vissza
Témakörök
- Idegennyelv > Idegennyelvű könyvek > Angol > Természettudományok > Fizika
- Idegennyelv > Idegennyelvű könyvek > Angol > Természettudományok > Kémia
- Természettudomány > Fizika > Elméletek és törvények > Egyéb
- Természettudomány > Fizika > Idegennyelvű
- Természettudomány > Fizika > Társtudományok > Kémia
- Természettudomány > Kémia > Általános kémia > Általában
- Természettudomány > Kémia > Idegennyelvű
- Természettudomány > Kémia > Szerves kémia
- Természettudomány > Kémia > Társtudományok > Fizika